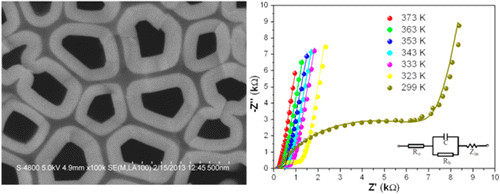
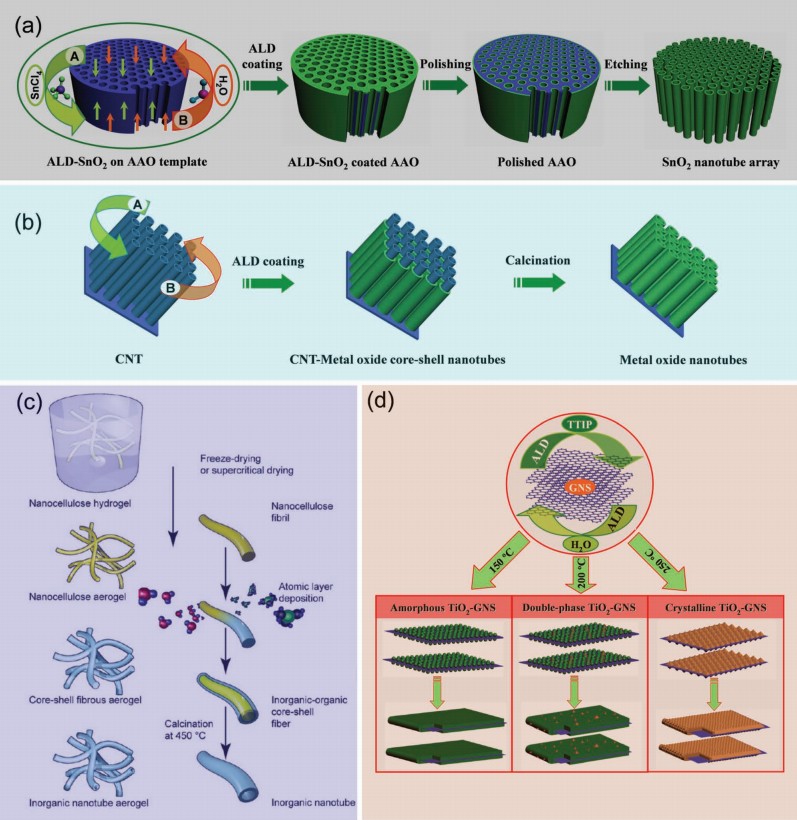
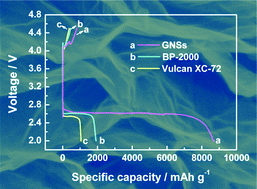
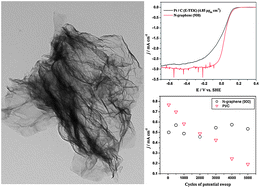
You are using a browser that is not standards-compliant
(possibly Netscape 4). The information on this Web site will be accessible to
you, but for a list of Web browsers that comply with the World Wide Web
Consortium standards, please visit our Web standards page.

Welcome to Dr. Sun's Nanomaterials and Energy Group
| Highlight | ||||||
|
|
Selected 20 typical papers from our group |
|
|


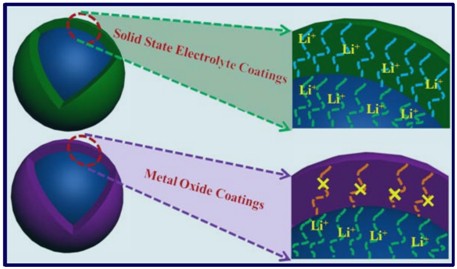
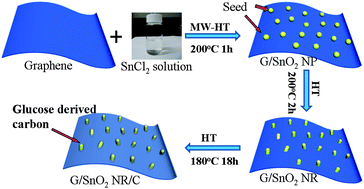
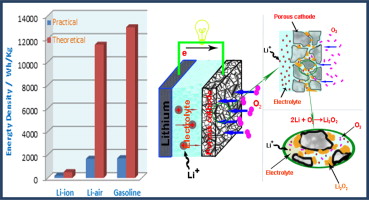
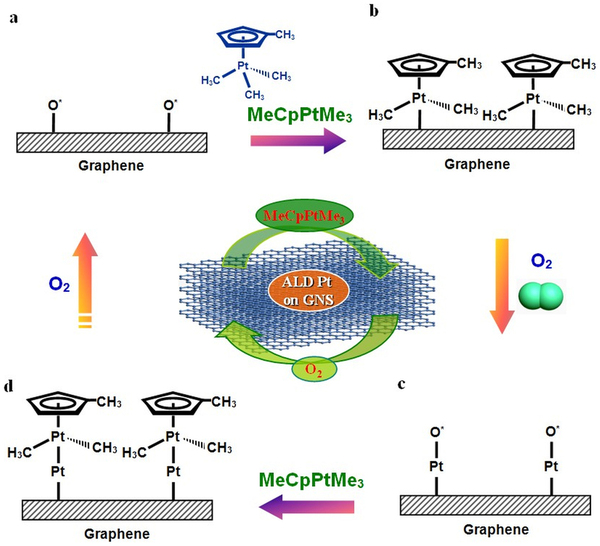
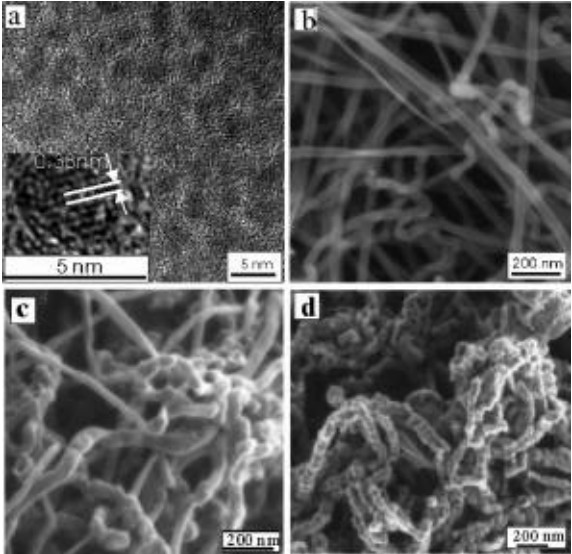
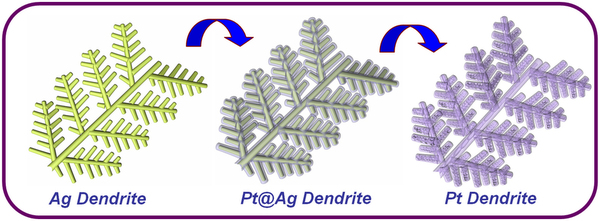 Controlling the morphology of Pt nanostructures can provide
opportunities to greatly increase their activity and stability.
Porous dendritic Pt nanotubes were successfully synthesized by a
facile, cost-effective aqueous solution method at room temperature
in large scale. These unique structures are porous, hollow,
hierarchical, and single crystalline, which not only gives them a
large surface area with high catalyst utilization, but also improves
mass transport and gas diffusion.
Controlling the morphology of Pt nanostructures can provide
opportunities to greatly increase their activity and stability.
Porous dendritic Pt nanotubes were successfully synthesized by a
facile, cost-effective aqueous solution method at room temperature
in large scale. These unique structures are porous, hollow,
hierarchical, and single crystalline, which not only gives them a
large surface area with high catalyst utilization, but also improves
mass transport and gas diffusion.


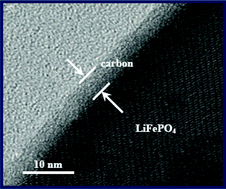
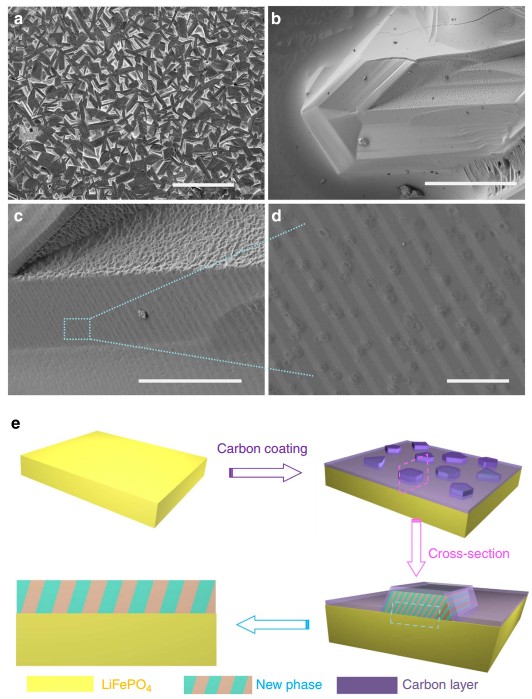
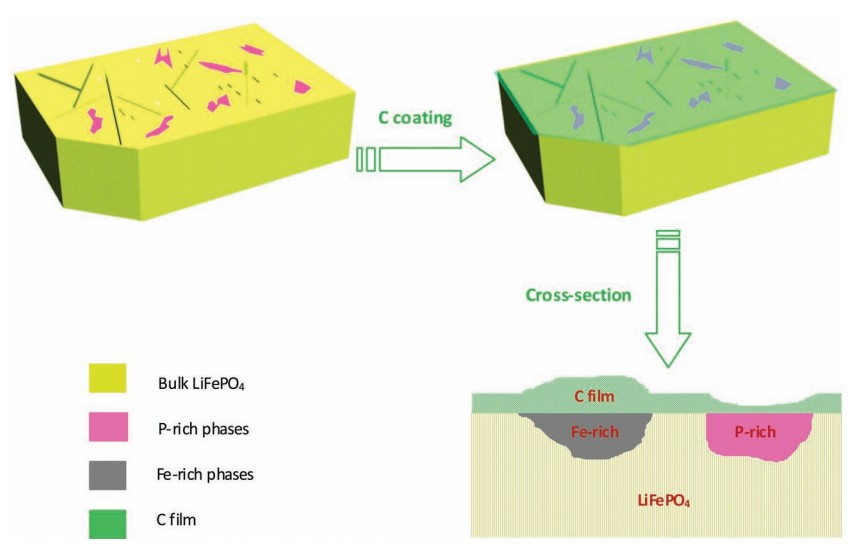 Carbon
coating is a proven successful approach for improving the
conductivity of LiFePO4 used in rechargeable Li-ion batteries. Different
impurity phases can be formed during LiFePO4 synthesis. Here, a direct
visualization of the impact of impurity phases in LiFePO4 on a carbon
coating is presented; they are investigated on a model material using
various surface-characterization techniques. By using polished ingot
model materials, impurity phases can be clearly observed, identified,
and located on the surface of the sample by scanning electron microscopy
(SEM), focused-ion-beam lithography (FIB), high-resolution transmission
electron microscopy (HR-TEM), and Raman spectroscopy. During the
carbon-coating process, the phosphorus-rich phase is found to have an
inhibiting effect (or no positive catalytic effect) on carbon formation,
while iron-rich phases (mainly iron phosphides) promote carbon growth by
contributing to more carbon deposition and a higher graphitic carbon
content. This finding, and the methodological evaluation here, will help
us to understand and reveal the influencing factors of impurity phases
on the basic carbon-deposition process to obtain high-performance
LiFePO4 material for future energy-storage devices.
Carbon
coating is a proven successful approach for improving the
conductivity of LiFePO4 used in rechargeable Li-ion batteries. Different
impurity phases can be formed during LiFePO4 synthesis. Here, a direct
visualization of the impact of impurity phases in LiFePO4 on a carbon
coating is presented; they are investigated on a model material using
various surface-characterization techniques. By using polished ingot
model materials, impurity phases can be clearly observed, identified,
and located on the surface of the sample by scanning electron microscopy
(SEM), focused-ion-beam lithography (FIB), high-resolution transmission
electron microscopy (HR-TEM), and Raman spectroscopy. During the
carbon-coating process, the phosphorus-rich phase is found to have an
inhibiting effect (or no positive catalytic effect) on carbon formation,
while iron-rich phases (mainly iron phosphides) promote carbon growth by
contributing to more carbon deposition and a higher graphitic carbon
content. This finding, and the methodological evaluation here, will help
us to understand and reveal the influencing factors of impurity phases
on the basic carbon-deposition process to obtain high-performance
LiFePO4 material for future energy-storage devices.